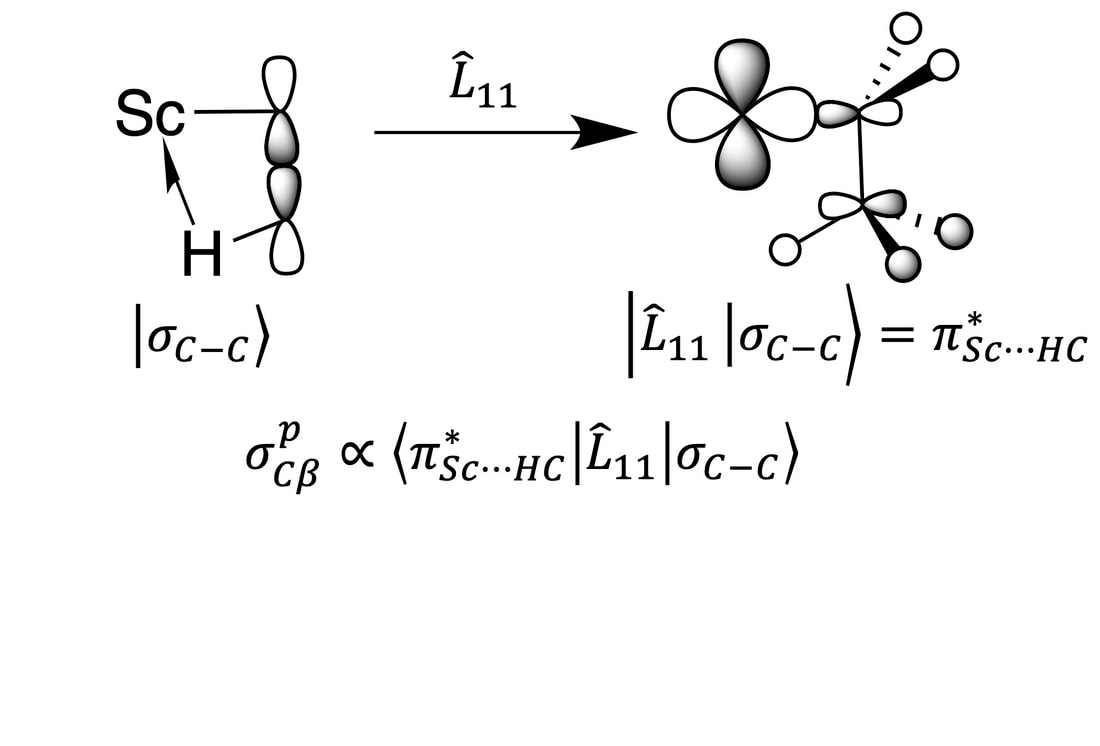Our Research: Broad Strokes
~90 % of all chemical syntheses in chemical industry include at least one catalytic step. To put this number into dollars and cents, catalysts contribute either directly or indirectly to ~35 % of global GDP (~$27 trillion). A vast majority of catalytic reactions use heterogeneous catalysts because they readily separated from reaction mixtures, can often be regenerated, and are generally easy to prepare by wetting a high surface area oxides with a solution of metal precursor. But this comes with a hidden cost, heterogeneous catalysts have poorly defined active site structures, ultimately resulting in less energy efficient reactions.
Our central goal is to generate active sites on surfaces that have well-defined structures amenable to structure-activity relationships. Our approach involves the preparation of high surface area materials containing characteristic surface sites that react with inorganic complexes to form metal sites with well-defined coordination spheres. This allows us to optimize the activity of heterogeneous catalysts based on the molecular structure of the active site, which we determine by spectroscopic techniques, and to study the key mechanistic steps in the catalytic cycle. Many of the active sites on our well-defined materials are characterized using solid-state NMR spectroscopy. For more information click on the Publications tab to see our recent papers.
Our central goal is to generate active sites on surfaces that have well-defined structures amenable to structure-activity relationships. Our approach involves the preparation of high surface area materials containing characteristic surface sites that react with inorganic complexes to form metal sites with well-defined coordination spheres. This allows us to optimize the activity of heterogeneous catalysts based on the molecular structure of the active site, which we determine by spectroscopic techniques, and to study the key mechanistic steps in the catalytic cycle. Many of the active sites on our well-defined materials are characterized using solid-state NMR spectroscopy. For more information click on the Publications tab to see our recent papers.
Polyolefin Plastics: Building them Up and Breaking them Down
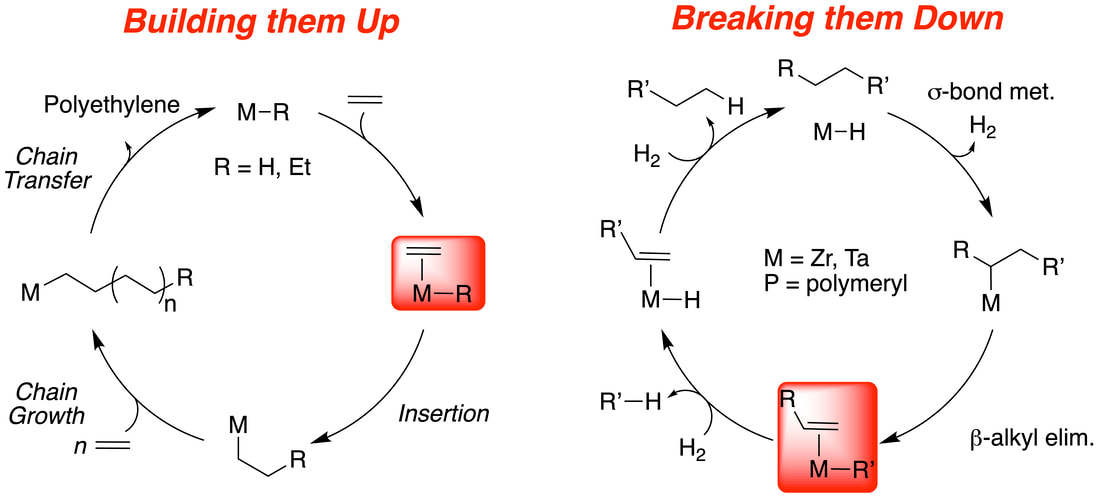
We are experts at generating well-defined catalysts for the polymerization of ethylene. This reaction is the largest industrial application of organometallic chemistry, and a vast majority of polyolefin plastics are synthesized using a heterogeneous catalyst. We study industrial mixture to determine how they self-assemble under reactor conditions (link) and we develop new methods to generate heterogeneous catalysts to access new plastics containing polar functionalities (link). The image above shows how to build up a polymer. An organometallic coordinates an olefin, inserts, and continues this process until the metal releases the polymer chain and restarts the cycle.
Though this reaction forms the basis of most of our groups expertise, we are not burying our heads in the sand to the enormous amounts of waste that plastic materials generate. How can we break down the polymer using chemistry? Our approach is simple, why not run the reaction in reverse? We do this by leveraging well-known organometallic chemistry to activate unreactive C-H bonds in polyethylene or polypropylene. This reaction triggers C-C bond cleavage to generate unstable intermediates that are intercepted by hydrogen to form mixtures of alkanes. We have shown this is viable using highly reactive tantalum hydrides (link and link). If you look at the two cycles in the above image carefully, the two red boxes look like very similar intermediates. This led to our current breakthrough: use an actual polymerization catalyst to break down the polymers, and it works (link). This result suggests that the broad industrial portfolio of olefin polymerization catalysts may also break down the waste polymers to facilitate a more circular plastics economy.
Though this reaction forms the basis of most of our groups expertise, we are not burying our heads in the sand to the enormous amounts of waste that plastic materials generate. How can we break down the polymer using chemistry? Our approach is simple, why not run the reaction in reverse? We do this by leveraging well-known organometallic chemistry to activate unreactive C-H bonds in polyethylene or polypropylene. This reaction triggers C-C bond cleavage to generate unstable intermediates that are intercepted by hydrogen to form mixtures of alkanes. We have shown this is viable using highly reactive tantalum hydrides (link and link). If you look at the two cycles in the above image carefully, the two red boxes look like very similar intermediates. This led to our current breakthrough: use an actual polymerization catalyst to break down the polymers, and it works (link). This result suggests that the broad industrial portfolio of olefin polymerization catalysts may also break down the waste polymers to facilitate a more circular plastics economy.
Direct Conversion of Ethylene to Propylene
|
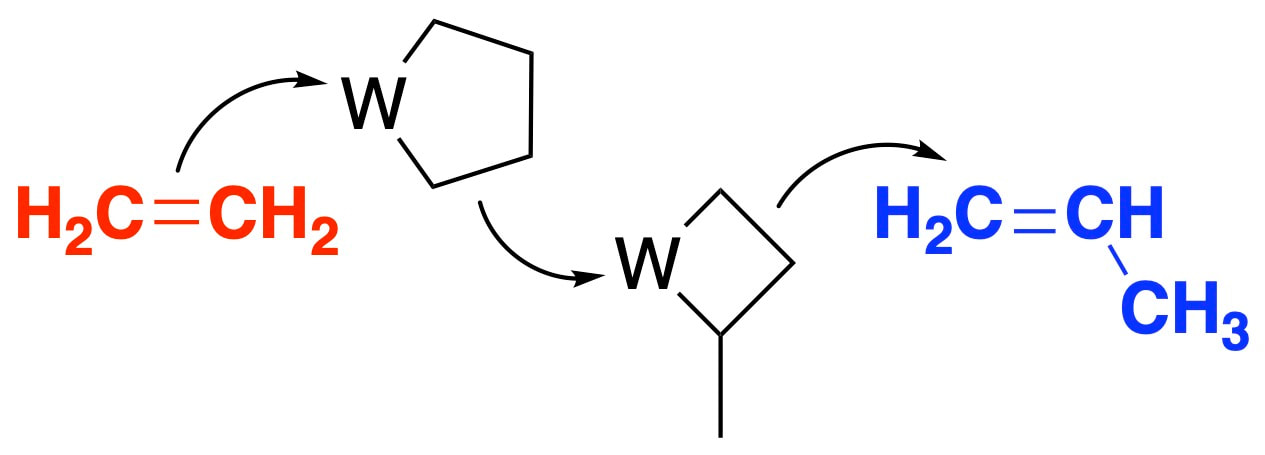
Propylene is a critical feedstock chemical produced on very large scales using steam cracking or dehydrogenation of propane. The third method involves olefin metathesis using a heterogeneous tungsten oxide catalyst. In close collaboration with Prof. Richard R. Schrock we are developing a new set of organometallic catalysts the directly convert ethylene to propylene. This reaction has several key benefits. First, ethylene is a widely available feedstock. Second, thermochemical analysis indicates that this reaction is far more energetically favorable than other known reactions to synthesize propylene. We are currently expanding the organometallic landscape for this reaction, our first paper on this project can be found here.
|
Ion-Pairing on Surfaces
|
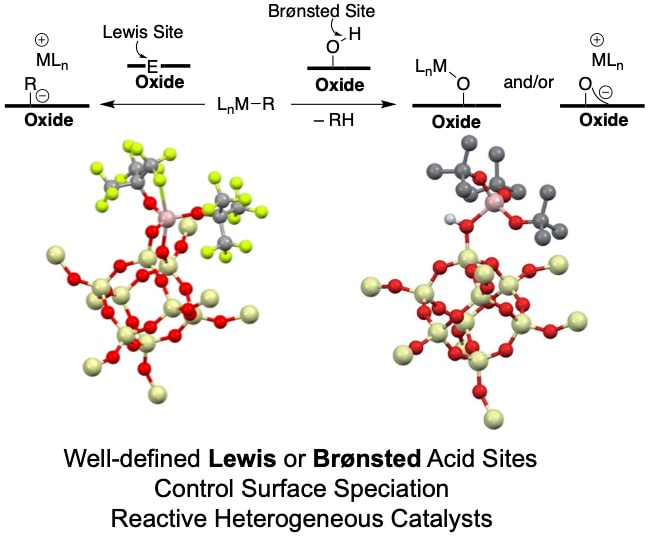
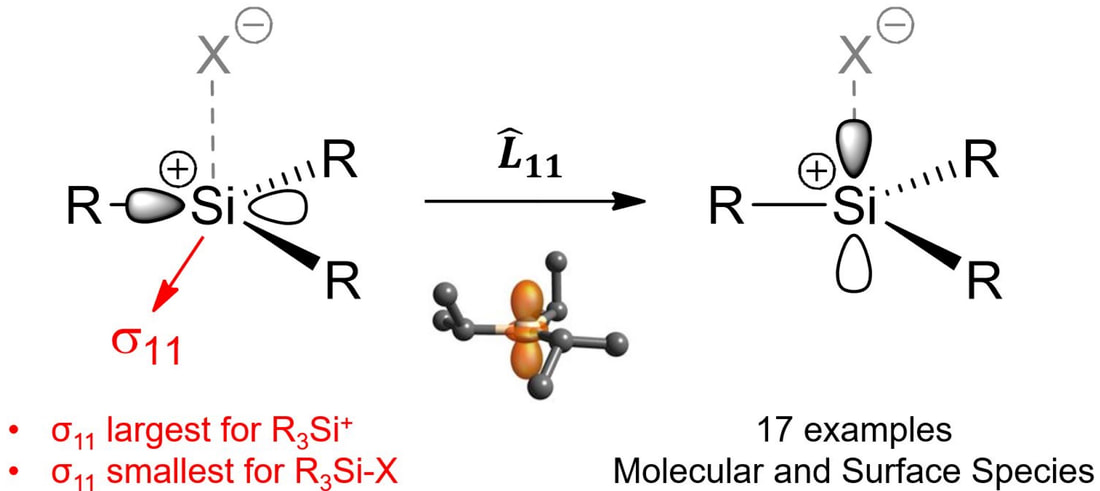
Virtually all of the catalysts made in our group are electrostatically tethered to a high surface area support as an ion pair. This allows us to generate highly reactive organometallic cations to achieve many of the challenging (or not so challenging) reactions we study. Over the past several years we developed several complementary methods to generate reactive cations on surfaces (perspective). For example, we installed very strong Lewis acid sites on an oxide to promote M–R abstraction reactions to form ion-pairs (link). We also showed that very strong Brønsted acid sites form on oxides when in contact with a very strong aluminum Lewis acid (link). This latter method is proving very versatile because we can generate rare examples of supported silylium ions, the silicon derivative of carbocations, that allow us to generate new reactive sites by halide abstraction methods that were previously unavailable to the surface community (link and link). We are actively pursuing this new method to generate a library of well-defined organometallic cations for new catalytic applications.
|
How do we characterize these materials?
|
The hardest part of any chemical endeavor is determining the structure of whatever it was you made. Life is even harder when doing our chemistry because diffraction methods are not viable since we spread our highly reactive surface species onto a high surface area support. We use solid-state NMR spectroscopy, by far the most powerful method to obtain molecular structure on these types of materials. All of the projects mentioned above involve a component of solid-state NMR spectroscopy. We often dig deep into the meanings of the line shapes and/or chemical shifts we obtain in these experiments, and on occasion we find ourselves studying interesting properties of molecular compounds with this method because there aren't many groups that can make the organometallic and study solid-state NMR properties. For example, we solved an ~40 year mystery showing that a classic organoscandium complex contains a 3-center-2-electron interaction, which was suspected but not detectable using diffraction nor solution NMR techniques (link). We also studied the origin of the silicon-29 NMR chemical shift in organosilanes and silylium-ions. Contrary to what is often written in textbooks, the chemical shift of these compounds has nothing to do with charge. Rather, appropriate alignment of the HOMO and LUMO in planar silylium ions results in large paramagnetic shift contributions, resulting in a downfield NMR chemical shift (link).
|
Interested in Joining?
Prospective Postdocs:
We are searching for a postdoctoral researcher. If you are interested send your CV (with 3 references) and brief description of your graduate research.
Prospective Undergraduate Students:
Undergraduate research is a great opportunity to gain experience in the lab, and highly encouraged for students interested in chemistry as a career. Interested students should contact Matt by email and also take a look at the UCR Undergraduate Research page.
Prospective Graduate Students:
Apply directly to the Chemistry Graduate Program at UC Riverside. [link] We are happy to discuss our research with prospective graduate students, but can not answer questions about admissions.
We are searching for a postdoctoral researcher. If you are interested send your CV (with 3 references) and brief description of your graduate research.
Prospective Undergraduate Students:
Undergraduate research is a great opportunity to gain experience in the lab, and highly encouraged for students interested in chemistry as a career. Interested students should contact Matt by email and also take a look at the UCR Undergraduate Research page.
Prospective Graduate Students:
Apply directly to the Chemistry Graduate Program at UC Riverside. [link] We are happy to discuss our research with prospective graduate students, but can not answer questions about admissions.